14/05/2024
14/05/2024
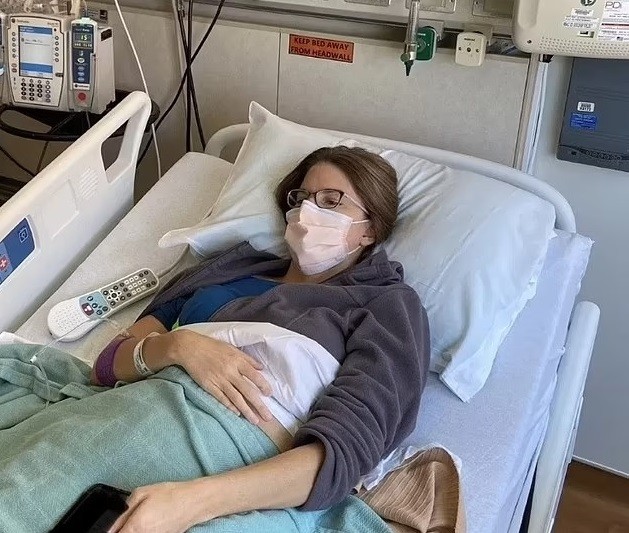
NEW YORK, May 14: Brianne Dressen, a former teacher from Utah, has filed a lawsuit against AstraZeneca, the pharmaceutical giant, alleging that the company's COVID-19 vaccine trial left her "permanently disabled." Dressen, who participated in the US clinical trial of the AstraZeneca vaccine in 2020, claims to have developed a severe neurological condition after receiving the jab.
In court documents filed in Utah, Dressen accuses AstraZeneca of breaching its contract by failing to provide medical care for her side effects. Her lawsuit marks the first of its kind in the United States, where the British-made vaccine was tested in clinical trials but never approved for use.
Following the trial, Dressen experienced a severe sensation of pins and needles across her body, leading to a diagnosis of peripheral neuropathy—a condition causing numbness and pain due to damaged nerves. She attributes her condition to the vaccine and claims that AstraZeneca did not cover the cost of her medical care as promised in the contract.
According to Dressen, her illness has left her permanently disabled, rendering her unable to work or engage in athletic activities. She has incurred substantial medical bills and is seeking damages for emotional distress, lost income, transportation, and legal fees.
While the AstraZeneca vaccine has been credited with saving millions of lives during the pandemic, it has also faced scrutiny for potential side effects. The company recently acknowledged in court documents that the jab could cause blood clots in very rare cases.
AstraZeneca has declined to comment on ongoing litigation, but it maintains that the vaccine has an acceptable safety profile and has played a critical role in ending the global pandemic.
Dressen's lawsuit underscores the complex debate surrounding vaccine safety and highlights the challenges faced by individuals who experience adverse reactions. As the case unfolds, it raises questions about the accountability of pharmaceutical companies and the protection of clinical trial participants.


