11/11/2023
11/11/2023
An explosive report reveals that contaminated eyedrops, posing risks of infections and potential blindness, were pulled from pharmacy shelves. These products, sold under store brands at major retailers like CVS, Rite Aid, Walmart, and Target, originated from Kilitch Healthcare India Ltd., located in Navi Mumbai. The FDA issued a recall on more than two dozen eyedrop brands on October 25, citing unsanitary conditions at the Indian plant and positive bacterial test results from critical drug production areas.
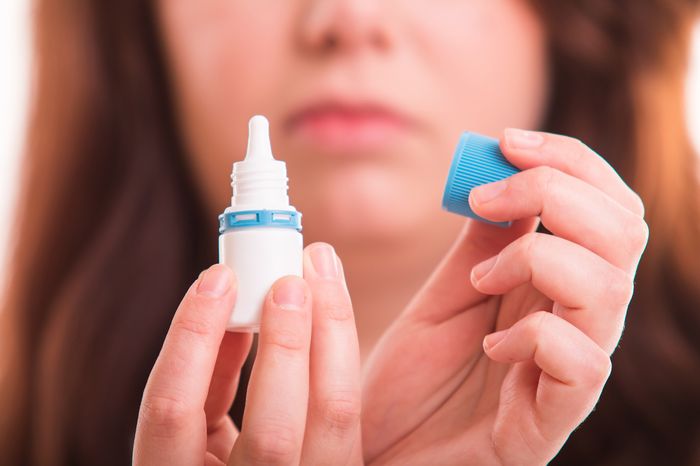
The government report disclosed instances of workers at the Indian factory walking barefoot or without protective gear in sterile areas. Shockingly, some were observed engaging in personal grooming amidst cleaning equipment, and others were found falsifying dates on product labels attesting to their sterility. The FDA, which proactively collaborated with retailers to remove the products before injuries were reported, urged consumers to cease using the affected eyedrops due to the risk of eye infections.
This incident marks the second time U.S. authorities discovered problems with Indian-made eyedrops. A prior case involved infections, deaths, and vision loss linked to another Indian company, EzriCare LLC and Delsam Pharma LLC. A lapse in FDA supervision allowed these products to reach the U.S. market. Despite India's prominence in pharmaceutical manufacturing, the FDA's limited power to enforce recalls has raised concerns about the safety of over-the-counter medicines, with the Kilitch factory banned from sending more eyedrops to the U.S. following an October 23 inspection.