30/01/2024
30/01/2024
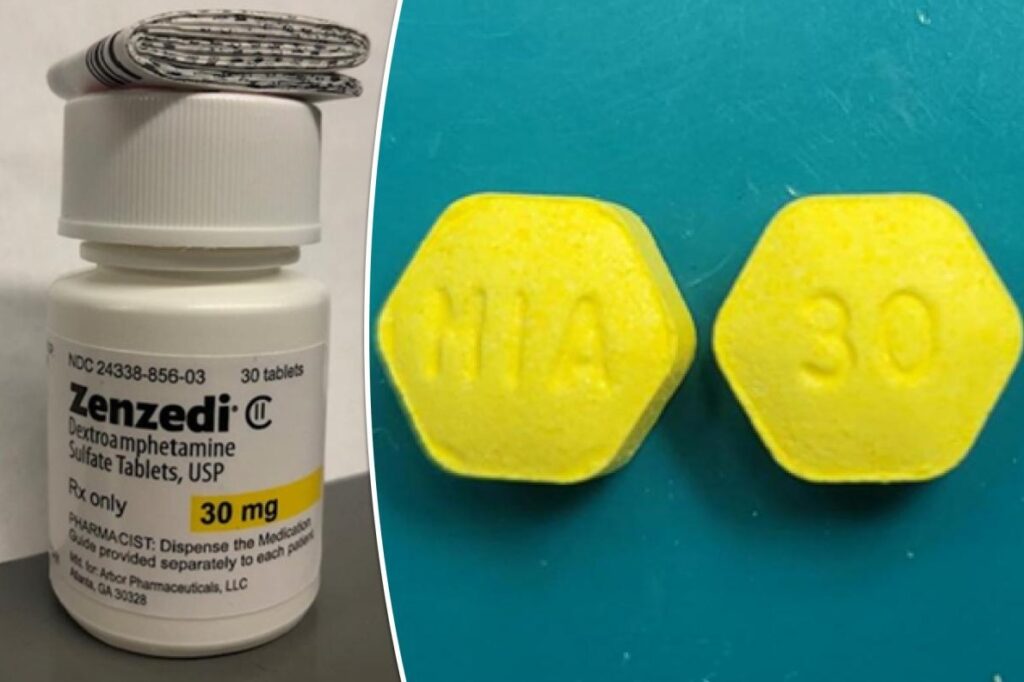
NEW YORK, Jan 30: Azurity Pharmaceuticals has initiated a recall of its attention deficit hyperactivity disorder (ADHD) and narcolepsy medication, Zenzedi, following the discovery of incorrect pills in a drug package. The recall notice, issued on Wednesday, specifies that one lot of Zenzedi 30 milligram tablets is being recalled after a pharmacist identified tablets of carbinoxamine maleate, an antihistamine, in a bottle labeled as Zenzedi.
This recall adds to the challenges faced by individuals with ADHD, as the nation is already grappling with a shortage of ADHD medications since October 2022, leading to increased confusion and unpredictability for those seeking essential medications.
Zenzedi contains dextroamphetamine sulfate, a stimulant used for treating ADHD and narcolepsy. In contrast, carbinoxamine maleate, the allergy drug mistakenly included in the Zenzedi bottles, is a sedative with effects opposite to a stimulant, as highlighted by the Mayo Clinic.
Individuals who inadvertently take carbinoxamine instead of Zenzedi face an elevated risk of accidents or injuries, along with potential side effects such as drowsiness, increased eye pressure, urinary obstruction, and thyroid disorder, according to the recall notice. The affected lot carries the identification F230169A and has an expiration date of June 2025.
Azurity assures that no reports of serious injuries related to the medication mix-up have been received. Consumers possessing the recalled medication are urged to return it to their pharmacy promptly. If adverse reactions occur, individuals should contact their healthcare provider. Additionally, problems can be reported to the FDA's MedWatch Adverse Event Reporting program.


